Valve Regulated Lead-Acid Batteries - VRLA
1. Traditional Lead-Acid Batteries ("vented" batteries or "open" batteries)
Vented lead-acid
battery gassing
At the end of lead-acid battery charging, gases are produced
at battery plates. The main problem is oxygen generation at the positive plate. Oxygen can recombine
at the negative plate but plates are separated with the liquid electrolyte. Oxygen diffusion inside
liquid is very slow. That is why only the small part of oxygen is recombined in the traditional
lead-acid batteries - the most part of it form bulbs rising to the surface of the electrolyte and
goes outside through the vents on the top of the battery.
So water (the part of battery electrolyte) is lost and periodically one needs to check the electrolyte
level in the battery and fill the battery with water if the level is low.

Filling traditional
lead-acid battery
with water
These traditional lead-acid batteries are called "open"
or "vented" because the battery volume is directly connected with the surrounding air and
any gas produced in the battery can flow outside. They are also called "flooded" because
electrolyte forms free liquid volume around battery plates.
Periodical electrolyte level checking is very unconvinient while
neglecting this check often results in battery damage. Is it possible to make lead-acid battery
maintenance-free?
2. The VRLA Idea
The main idea is to accelerate oxygen diffusion in lead-acid
battery to a level at which all the oxygen generated at the end of normal battery charging recombines
inside the battery. In this case the battery vent could be replaced with a self-sealing safety valve.
The valve would open only in emergency case (for example if charging current is several times larger then
normal) and would seal the battery under normal conditions.
The oxygen diffusion rate depend on several variables but changing
main of them: temperature and pressure, can accelerate diffusion of a few percent - it is not enough -
we need some orders of magnitude. Is it possible?
It is possible if we change the medium for the diffusion. The
diffusion in gas can be thousand times faster than diffusion in liquid. So if we replace some liquid
on the diffusion path by gas oxygen diffusion to accelerate. We need to place some gas cavities in the
liquid electrolyte and not allow them to rise to the electrolyte surface and dissappear. Magic?
Such lead-acid batteries do exist. They are called VRLA batteries
(Valve Regulated Lead-Acid). They are sealed when charging or discharging, can be used in different
positions (only charging in upside-down position is not recommended). There are safety, they do not
emit gas and they are almost maintainance-free. What is the cost of this happiness and how is it made?
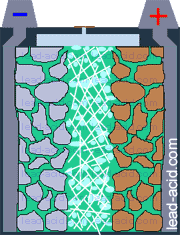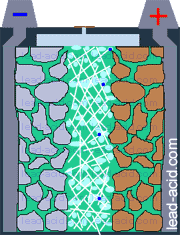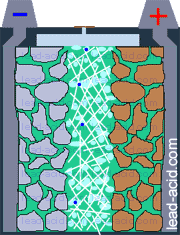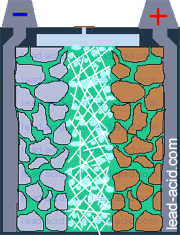
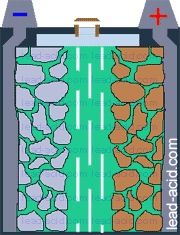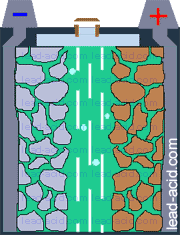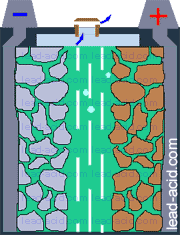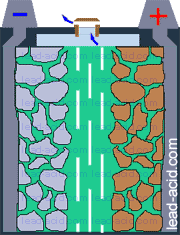
Oxygen migration
in VRLA battery
Two VRLA technologies were suggested so far - AGM and gel.
3. AGM - Absorptive Glass Mat
Good old porous plastic separator of the usual lead-acid
battery could be replaced with mat made of very thin glass fiber. This nonconducting mat serves as a
separator, fills the entire volume between battery plates and formes a highly porous medium. It
absorbs the main part of the electrolyte and still has some empty pores inside: cavities filled with
gas - not liquid. These bubbles can't rise to the electrolyte surface due to surface tension forces and
form the excellent paths for oxygen diffusion. The AGM battery electrolyte is the same that is used in
vented lead-acid battery - sulfuric acid H2SO4 mixed with water.
All the oxygen generated at the positive plate of lead-acid battery
diffuses partly through liquid and mainly through gas to the negative plate where it recombines with
the set of reactions:
O2- + 2Pb -> 2PbO
2PbO + 2H2SO4 -> PbSO4 + 2H2O
2PbSO4 + 4H+ + 4e- -> 2Pb + 2H2SO4
If we summarise these reactions we receive a very simple and logical overall
reaction:
It means that all the oxygen generated at the positive plate recombines
with all the hydrogen generated at the negative plate of lead-acid battery and forms water. So no water is
lost, no water filling is needed, no electrolyte level checking is needed - AGM lead-acid battery is
maintainance-free. So we can seal it with a safety wall, put it in the place and left working. It works on
it's side and even can be discharged upside-down.
4. Gel technology
Another possibility of immobilizing gas cavities is realised by immobilizing
of electrolyte itself. Sulfuric acid electrolyte is mixed with the grains of silica (silicon dioxide) with extremely
large surface area to form gel - a jelly-like soft and elastic substance that is something between liquid and solid.
From the chemical side it is the same sulfuric acid electrolyte - all reactions native for lead-acid battery
take place in a battery with gelled electrolyte. From the physical side it is not liquid that is why any gas bulb
or gas cavity remains at the place where it was formed. Gas cavities unite forming long 3-d cracks that sometimes
extend from one battery plate to another. This is the ideal path for oxygen diffusion.
The same oxygen migration mechanism is realized in gel VRLA batteries. The same
oxygen reconbination reactions take place at the negative plate. The same is the result - no water loss, no battery
filling and no service.
VRLA -
nonspillable battery
5. VRLA - Nonspillable battery
Two VRLA technologies lead to same result: electrolyte is fixed.
It is absorbed in the glass mat separator of AGM battery or immobilized by gelling in gel battery. So both types
of AGM batteries are nonspillable. If you turn the battery upside-down your pants are safe. Even if the AGM battery
case is damaged - electrolyte stayes inside.
So AGM battery can be used in any position, only battery charging in upside-down
position is not recommended fo safety. These batteries can be used with common ventilation systems - no need to take
off dangerous hydrogen-oxygen mixture. Only in case of battery charger falure gases can come off the battery.
6. AGM and gel batteries: positives
The main advantages of VRLA batteries (both AGM and gel) compared to traditional
lead-acid batteries are as follows.
- VRLA batteries are maintenance-free or very close to it.
- VRLA batteries do not emit gases and can be used without special ventilation.
- AGM and gel batteries are nonspillable - there are easier to transport and maintain; batteries can be used in any position.
- VRLA batteries have prolonged battery life under float charge.
- VRLA batteries have prolonged storage life - self discharge rate is 1-3% of battery capacity a month.
7. AGM and gel batteries: negatives
Both VRLA battery types have some disadvantages compared to traditional
lead-acid batteries.
- VRLA batteries are more expencive then traditional vented batteries.
- VRLA batteries are very sensitive to the violation of charge volage or current. They can lose water if
charge current or voltage are larger then recommended and there is no possibility to add water to the battery
- the battery is sealed.
- That is why VRLA charging voltage must match the battery temperature - automatic temperature
compensation must be used.
- Usually the maximum discharge of VRLA batteries is smaller compared to traditional batteries of the same
capacity.
8. AGM and gel batteries: differences
Both VRLA battery types are more or less similar. Similar in applications
and maintainance. Similar in main technical characteristics. Only some points should be mentioned discussing
differecies of AGM and gel batteries.
- Maximum charge and discharge currents for AGM battery are usually larger than gel battery currents.
- AGM battery capacity usually declines with increased current more slow than gel batery capacity.
- That is why VRLA charging voltage must match the battery temperature - automatic temperature
compensation must be used.
- Usually the maximum discharge of VRLA batteries is smaller compared to traditional batteries of the same
capacity.
9. Applications of VRLA batteries
Modern VRLA batteries are more expencive than old-style lead-acid batteries.
So they are firrst used in applications sensible to main advantages of AGM ang gel batteries: low service
costs and absence of any leakage from the battery. The main applications are: UPS, reserve power in communications,
luxary cars and bikes, marine.
|