Lead Acid Battery history
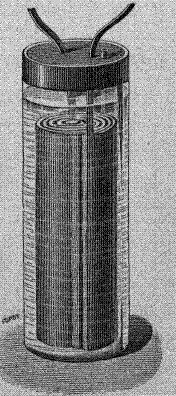
First lead-acid cell by Planté was made "by rolling two
long, wide lead plates into a coil,
separated one from the other by a thick cloth and then immersing them in a glass jar full
of water acidulated with a tenth part sulphuric acid".
1. Secondary cell idea and Planté's cell
Lead acid battery was the first known type of rechargeable battery.
It was suggested by French physicist Gaston Planté in 1860 (Comptes, rendus, t. L, p. 640. Mars 1860)
for means of energy storage.
Gaston Planté wrote in his "Storage of Electrical Energy":
"Secondary currents were observed at the beginning of this century (19-th sentury, of course),
shortly after the discovery of the Voltaic Battery. Gautherot, a French Scientist, was the first to
discover, in 1801, that platinum or silver wires which had been used to decompose saline water by this
battery, possessed the property, after having been cut off from the battery itself, of giving an electric
current of short duration."
So Planté's idea was very simple (as any other idea after we know it):
to use these extremely small "secondary currents" for energy storage. In other words - to make
a secondary cell or rechargeable cell.
Years of testing different materials lead finally to what? To lead
and sulfuric acid and to forming technology.
First lead-acid cell had plates made of pure lead and initially
had zero capacity. Some capacity arrived only after charging - applying electric current to the cell
during some time in order to oxidize the surface of the positive plate. The battery capacity after
first charge was very small, because of rather small active surface. To increase capacity cell
was discharged and charged several times. After such type of cycling (it is called "forming")
the surface of the negative plate is covered with porous lead, while the surface of the positive
plate is covered with porous peroxidized lead. The surface area of such high porous structures is
very large, so large is the battery capacity.
From Planté's time till now lead-acid cell consists of two
electrodes (plates) immersed in sulfuric acid electrolyte. The active material of the negative plate is lead
(Pb), while the active material of the negative plate is peroxidized lead (PbO2).
2. Faure's cell
Further lead-acid battery improvement was made by French chemical engineer
Camille Alphonse Faure at the end of 19-th sentury. In order to make battery forming shorter he covered
two lead strips with minium (lead oxide, PbO) and put them separetely to two felt envelopes. Then these
two envelopes with identical lead strips inside were immersed to sulfuric acid electrolyte as in Planté's cell.
Lead strips (future battery plates) were connected to a primary battery. After some time diring which current was
flowing through the cell lead oxide on both plates had changed. It reduced to spongy metallic lead on the
negative plate and oxidized to lead peroxide (PbO2) on the positive plate.
The advantages of Faure's cell were the large active surface and the shortened
forming time. The idea of using of PbO as a primary product for lead-acid battery production is the essence of
modern lead-acid batteries manufacturing. All pasted lead-acid batteries are produced using PbO containing paste.
To make a battery cheaper and lighter lead strip swere were perforated to form plate grids - the paste
containig element of a modern lead-acid battery plate.
In short time Faure's battery characteristics were improved. The energy density
increased several times, batteries became lighter and more compact.
One of the drawbacks of Faure-style battery is the extreme sensitivity to deep
discharges. Once discharged below allowed voltage the battery loses part of active material when pieces of paste
fall down to the bottom of the battery jar. The other imperfection is the loss of electrolyte due to overcharge.
Both these weaknesses lead to a shortened battery life. And both them were more or less fixed in sentury 20-th.
3. Lead acid batteries in XX century
Active material loss due to plate-shedding can be eliminated or reduced
by pinching active material with some porous substance. This was fulfilled in tubular-plate batteries with
the help of porous tube containig active material with current-collector and in VRLA betteries by squeezing
active mass with separator.
Awater loss problem (losing a part of electrolyte due to overcharge)
was solved in valve-regulated lead-acid batteries (VRLA) by utilising of oxygen recombination mechanism.
All gases produced inside VRLA battery recombine inside and the battery is sealed under normal conditions.
active mass with separator.
So what do we have now? Now we have modern lead acid batery - the cheapest, the
most wide spread, the most powerful type of rechargeable cell and thousands of electro-chemical engeneers
thinking how to make it better.
|